Background
B-cell acute lymphoblastic leukemia (B-ALL) is a genetically heterogeneous group of acute leukemia with novel subtypes emerging. Since we first reported a novel subgroup B-ALL with half cases possessing CXCR4 truncated mutations and abnormal expression of CDX2 in all cases, the genomic features were further elucidated by different research groups that concurrent focal deletions lead to both upstream enhancer hijacking by CDX2 and UBTF::ATXN7L3 fusion transcript. But the molecular characterization and underlying mechanism beyond the novel subtype B-ALL remain ambiguous.
Methods
In order to comprehensively elucidate the molecular features, we adopt whole genome sequencing to reveal the genomic abnormalities, including snv, indel, copy number variant, and structural variation. Then, the signaling transduction and functional effect of differentially expressed genes were conducted by transcriptome analysis. Furthermore, we constructed a weighted gene co-expression network (WGCNA) and transcriptional regulons network to unravel the hub genes or transcriptional factors in the subtype.
Results
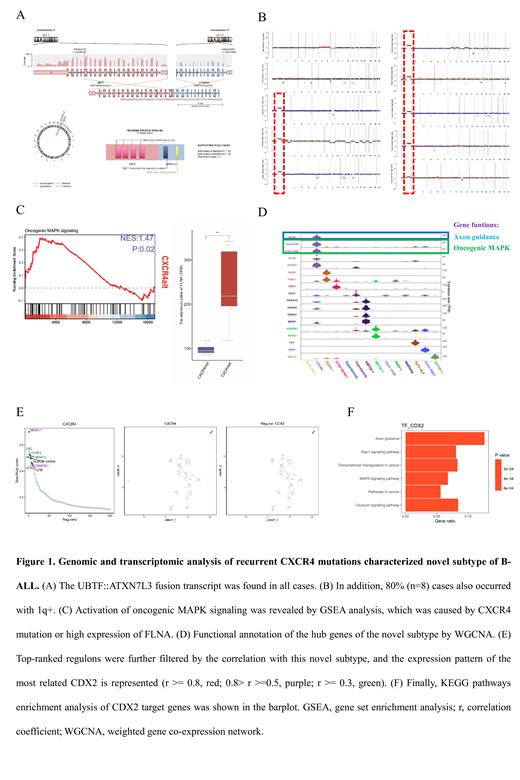
We retrospectively identify 2.4% (n=12) cases belonging to a novel subtype among 503 B-ALL cases with available transcriptome data by unsupervised clustering, and this subtype is characterized by CXCR4 truncated mutations or high expression of FLNA which could enhance CXCR4 signaling. Among these patients, only one male was affected showing a strong sex tendency towards females. Uniformly, all cases harbor both focal deletions encompassing between PAN3 and FLT3 located upstream of CDX2, and between UBTF and ATXN7L3, unraveled by whole genome sequencing (n=10). The focal deletions lead to overexpression of CDX2 by enhancer hijacking of upstream PAN3 and fusion transcript UBTF::ATXN7L3, respectively. In addition, 80% (n=8) cases also occurred with 1q+ which is regarded as a factor contributing to poor prognosis in leukemia and lymphoma. Transcriptomic analysis of the differentially expressed genes (DEGs) for this subtype confirmed the critical role of oncogenic MAPK signaling. Enhanced MAPK signaling was further supported by the function of the hub genes including KIAA1549 and KIAA1549L, which belong to oncogenic MAPK signaling, derived from WGCNA analysis. According to the functional enrichment analysis of DEGs, axon guidance was the only significant enriched KEGG pathway. The hub genes NTN3 from WGCNA analysis and regulon CDX2 both confirmed the functional involvement of axon guidance in this subtype. Furthermore, we prospectively identify 8 more cases by RT-PCR screening of the UBTF::ATXN7L3 transcript. Totally, twenty patients have been found in our cohort, and interestingly, there exist two cases among this subtype accumulated with multiple subclones characterized by different CXCR4 mutations, which strengthen the genotoxic stress over CXCR4 signaling.
Discussion
Although genomic characterization of this novel subtype has been revealed by our and others' research. The functional effect and underlying mechanism have never been elucidated. Here, we emphasize that enhanced CXCR4 signaling in this subtype may act as an upstream driver event, and it could be supplied as a therapeutic target.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal